The second of the Three Little Pigments goes by the name of carotenoid.
The chemical structures of carotenoids are based on a long zigzag chain of carbon and hydrogen atoms, frequently with a six-membered ring at one or both ends. Here are the structures of three abundant leaf carotenoids and an unusual one that appears during winter senescence of box leaves.
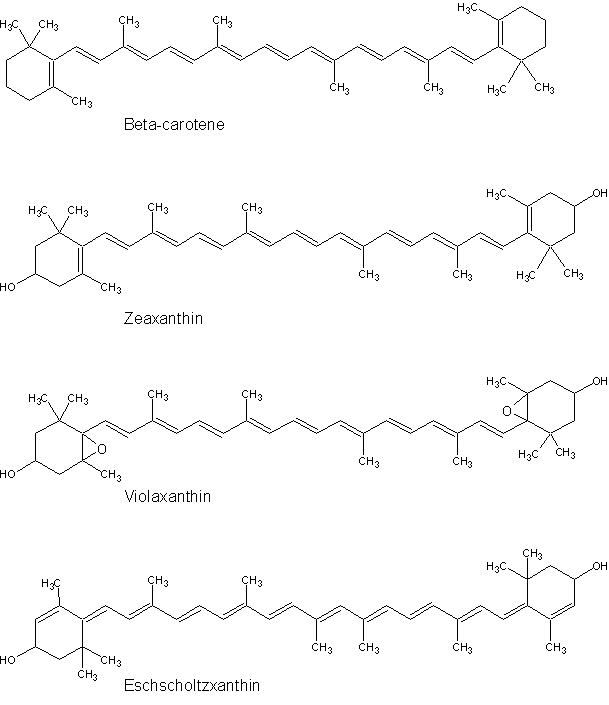
The presence, structure, and chemical modification of these rings determine the color of the carotenoid, which could be anything from yellow through orange to bright red.